Equivalent pharmaceuticals
An equivalent drug is a medicine that contains the same amount of active ingredient and has the same bioavailability as a branded drug whose patent has expired.
Branded medicines are protected by patents for several years to support the company that has invested heavily in research and development. At the end of this protected period, other pharmaceutical companies can produce the drug and sell it at more affordable prices.
Generic of equivalent



Bioequivalence
Two medicinal products are considered bioequivalent if their bioavailabilities, which is the quantity and rate at which the active ingredient is released and becomes available in circulation, are equivalent.
In practice, the concept of a generic medicine is based on the assumption that, in the same subject, an equal temporal pattern of the plasma concentration of the active substance results in an equal concentration of the substance at the site of action, and therefore, an equal therapeutic effect.
For this reason, the granting of Marketing Authorization (AIC) for a generic medicine by the Italian Medicines Agency (AIFA) is based on demonstrating the quality of the product and its bioequivalence compared to the originator medicine.
What the law says
The definition of the concept of generic medicine is contained in Article 10, Paragraph 5, Letter b of Legislative Decree 219 of 2006, which reads as follows: “generic medicine: a medicine that has the same qualitative and quantitative composition of active substances and the same pharmaceutical form as the reference medicine, as well as bioequivalence with the reference medicine demonstrated by appropriate studies of bioavailability.”
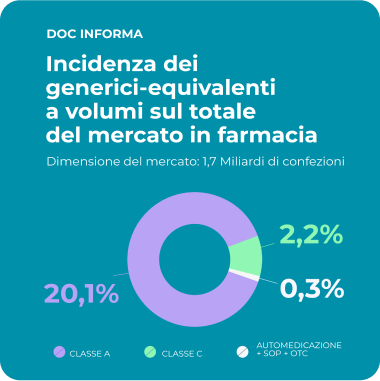
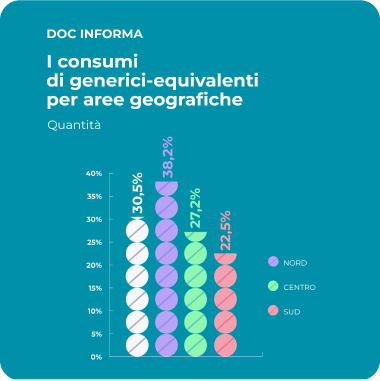
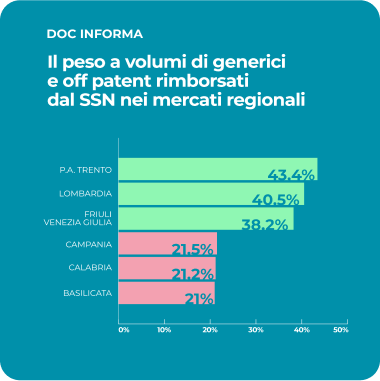
The Social Value of Equivalent Medicine
Why it costs less
A generic drug costs less than the originator because the investments made in research during the monopoly period granted by the patent have already been recovered.
It is well-known that demonstrating the therapeutic efficacy and safety of a new drug requires testing it on hundreds or even thousands of subjects. This testing takes months and incurs high costs.
The company that markets a generic medicine, however, is exempt from demonstrating therapeutic efficacy because, if the active ingredient reaches the same blood levels achieved by the originator medicine (i.e., if it is bioequivalent), it also presents the same therapeutic efficacy. Demonstrating bioequivalence compared to a drug with known efficacy and safety requires much less time and cost, allowing the generic drug to be marketed at a lower price.
