Quality
Project quality
Our main objective has always been to guarantee generic medicinal products with proven efficacy and constant characteristics, and that are easy for people to use. Quality is our way of doing business, which we apply from the very start of a product’s lifecycle and for its entire duration, with the constant monitoring of each and every phase of the process.
Our commitment to excellence continues, in addition to stringent controls, with a conscious decision to stand by pharmacists and physicians operating in the community by providing them with high-quality products. Because it is only together that the best results can be achieved.
Supply chain quality
Every day, we draw on the best starting materials, production systems and distribution chains to guarantee efficacy and efficiency in all stages of the manufacture and distribution of each and every product. The monitoring of the entire system is guaranteed, with on-going controls, by a great team of highly-specialised and qualified individuals.
For all our medications, we choose to use raw materials, production plants, and distribution chains of excellence, mainly in Italy and Europe.
DOC does not own production facilities; in fact, for the production of its medications, it relies on the best CDMOs (Contract Development and Manufacturing Organizations) in Italy and Europe.
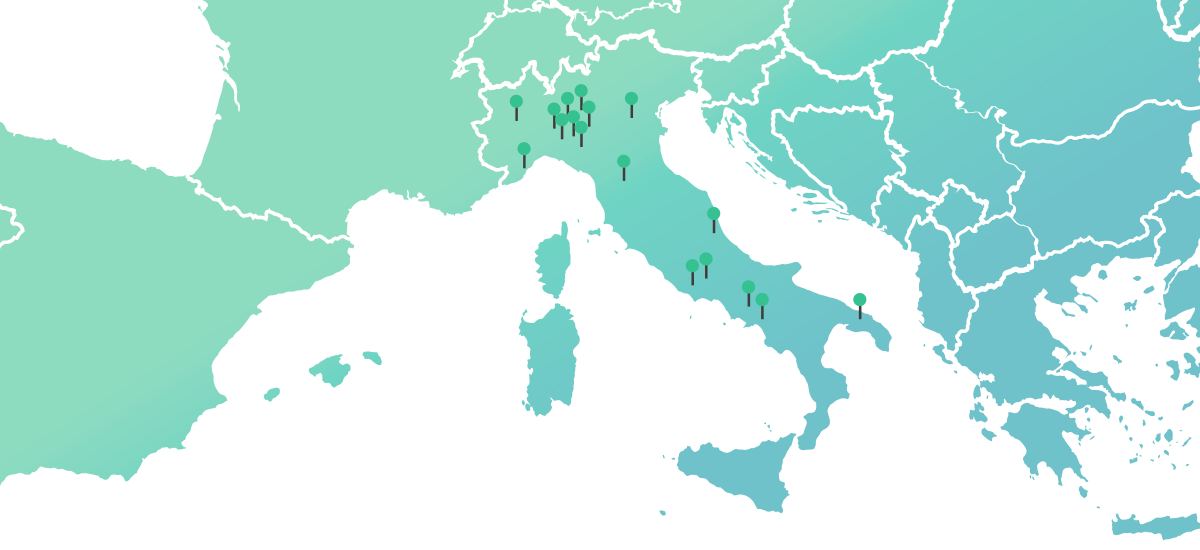
DOC all over the world
69 manufacturing sites in Europe*
0%

0%

5 manufacturing sites outside Europe*
*% per N packages FY 2023 internal data source
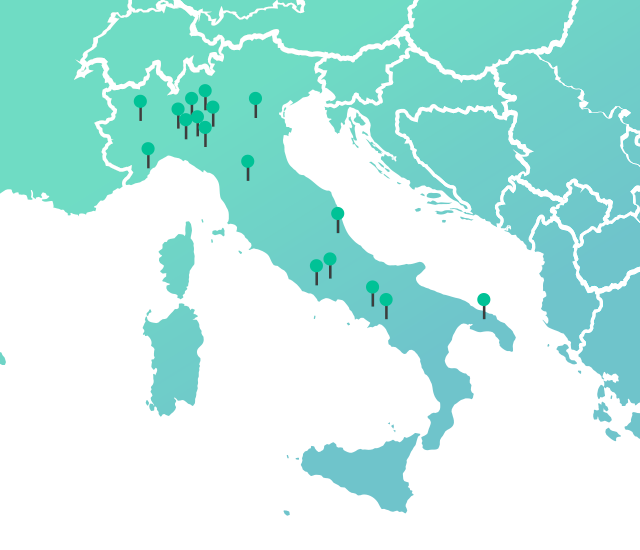
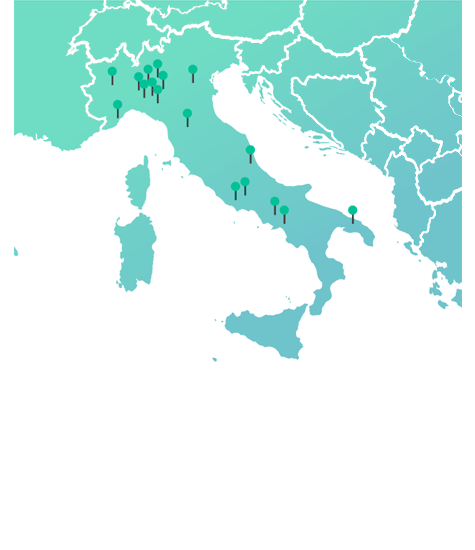
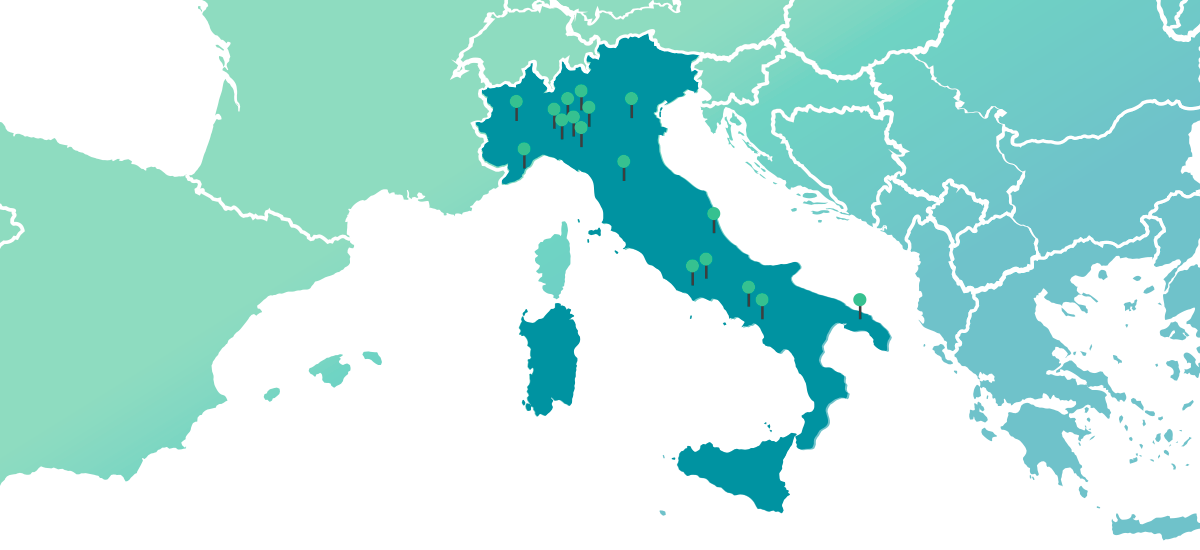
DOC in Italy
*% per N packages FY 2023 internal data source
0%* with 39 manufacturing sites
*% per N packages FY 2023 internal data source